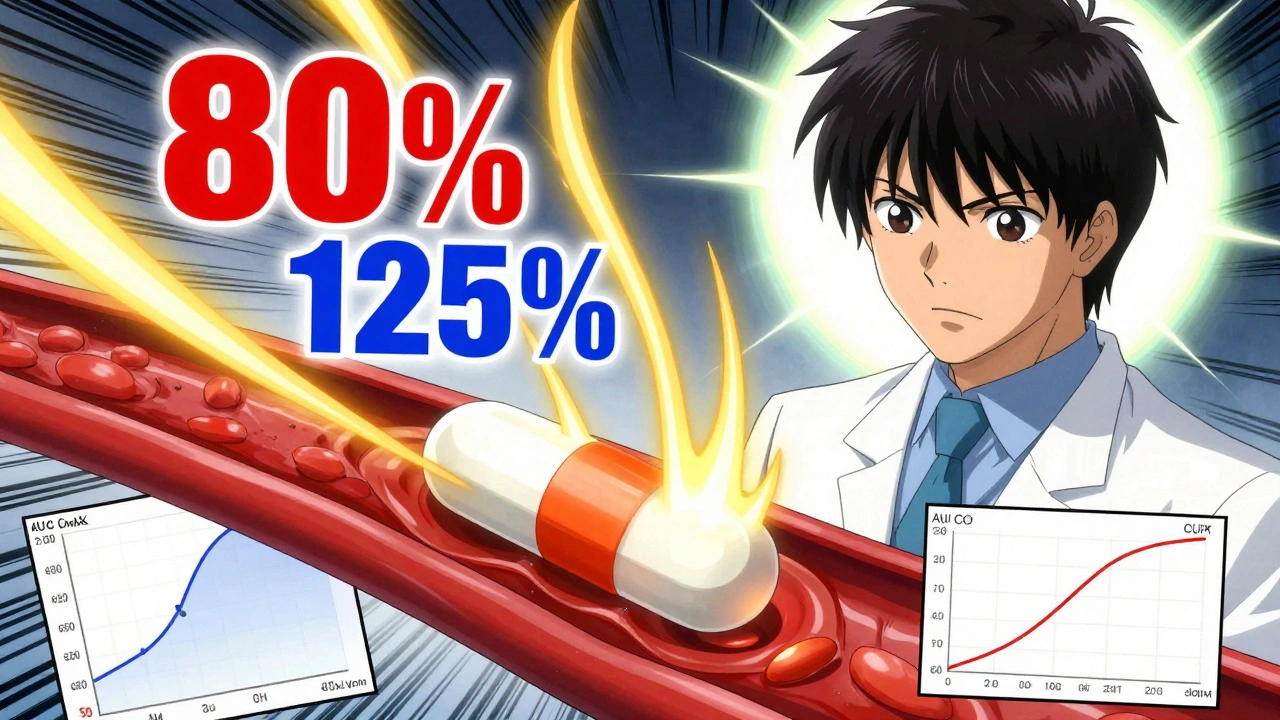
80-125 Rule: What It Means for Generic Drug Approval and Your Prescription Costs
When you pick up a generic pill, you expect it to work just like the brand-name version. That’s where the 80-125 rule, a FDA standard that defines acceptable bioequivalence between brand and generic drugs. Also known as the 80% to 125% rule, it ensures that the amount of active ingredient your body absorbs falls within a tight range compared to the original drug. This isn’t just bureaucracy—it’s the reason your blood pressure med, your insulin, or your antibiotic still does its job even when the label changes.
The bioequivalence, the measure of how quickly and completely a drug enters your bloodstream is the core of this rule. For a generic to be approved, its absorption rate must be between 80% and 125% of the brand drug’s. That means if the brand releases 100 units of medicine into your blood over time, the generic can release anywhere from 80 to 125 units—and still be considered the same. It’s not about identical pills. It’s about identical results. This standard applies to nearly all oral medications, from metformin to lisinopril, and it’s backed by real-world studies tracking thousands of patients. The FDA, the U.S. agency that regulates drug safety and approves generics doesn’t just accept claims—it requires labs to prove it with blood tests, often using healthy volunteers under controlled conditions.
Why does this matter to you? Because if a generic doesn’t meet the 80-125 rule, it could be too weak to work—or too strong and cause side effects. That’s why recalls happen. That’s why some people feel different on a new generic batch. The rule exists to prevent that. It’s also why the first generic to hit the market after a patent expires can charge a premium for 180 days—it’s the only one proven to meet this standard before others catch up. And that’s how your prescription costs drop over time. You’re not getting a cheaper version. You’re getting an equally effective one, verified by science.
Some people worry that generics are cut corners. But the 80-125 rule is the guardrail. It’s why doctors in Europe, Asia, and the U.S. trust generics. It’s why hospitals use them. And it’s why you can save hundreds—or thousands—over a lifetime without risking your health. Below, you’ll find real posts that dig into how this rule shapes drug approvals, why some generics fail, how age and sex affect bioequivalence, and what to do if your medication suddenly feels different. These aren’t theory pieces. They’re practical guides written by people who’ve seen the system up close.