When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how do regulators know it’s truly the same? The answer lies in a statistical rule most people have never heard of: the 80-125% rule. It’s not about how much active ingredient is in the pill. It’s not about purity. It’s about how your body absorbs the drug - and whether that absorption is close enough to the original to keep you safe and healthy.
What the 80-125% Rule Actually Means
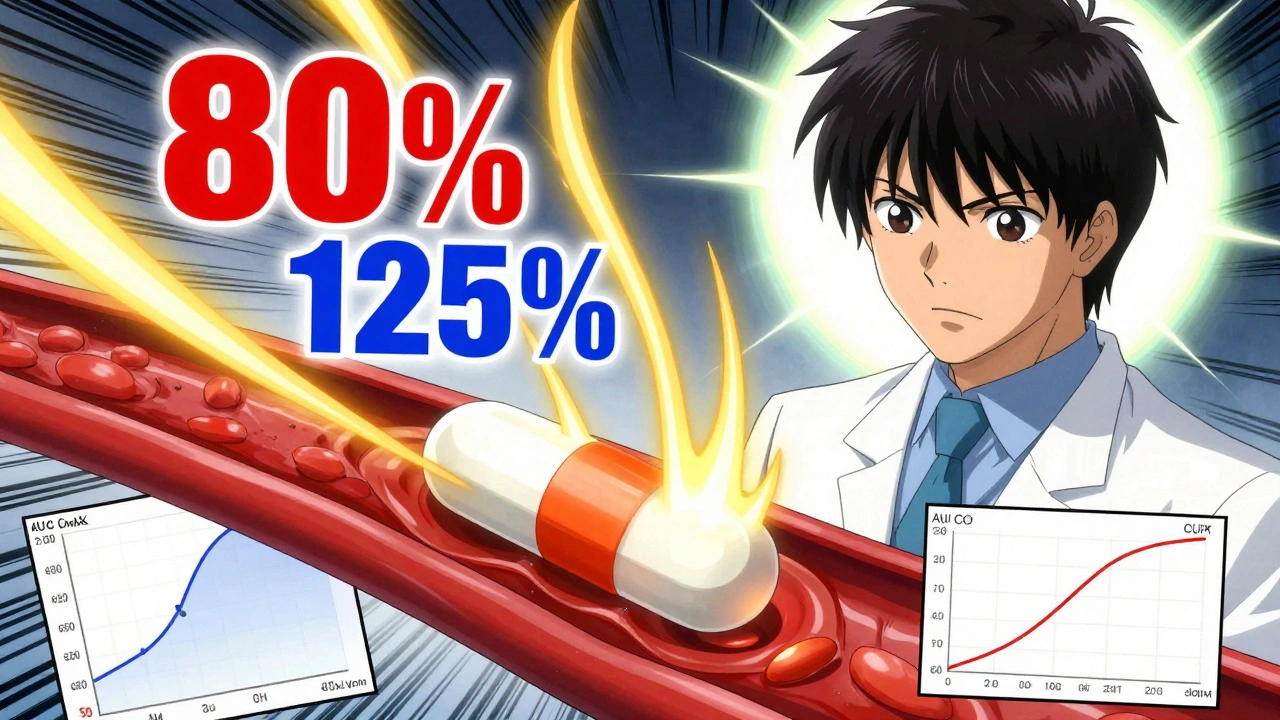
The 80-125% rule is the global standard for proving that a generic drug behaves the same way in your body as the brand-name version. It’s not a tolerance for how much drug is in the tablet - that’s tightly controlled too, usually within 95-105% of the label claim. This rule looks at what happens after you swallow it.
Two key numbers matter: AUC and Cmax. AUC, or Area Under the Curve, measures total drug exposure over time - basically, how much of the drug gets into your bloodstream and stays there. Cmax is the highest concentration reached. Both are measured in clinical studies using blood samples from healthy volunteers.
Researchers compare the average AUC and Cmax of the generic drug to the brand-name drug. But here’s the twist: they don’t use regular averages. They use geometric means, because drug levels in blood don’t follow a normal bell curve - they follow a log-normal curve. So they take the logarithm of the numbers, compare them, then convert back.
The rule says: the 90% confidence interval of the ratio of these geometric means must fall entirely between 80% and 125%. That means if the generic delivers 85% to 118% of the brand’s exposure, it passes. If it’s 79% or 126%, it fails - no matter how small the difference.
Why 90% Confidence Interval? Not 95%
You might wonder why it’s a 90% confidence interval instead of the more familiar 95%. It’s intentional. A 90% CI allows for 5% error on each end - 10% total. That’s the regulatory sweet spot: enough certainty to protect patients, but not so strict that it blocks good generics from reaching the market.
Think of it like this: if you used a 95% CI, you’d be demanding near-perfect matching. But drug absorption varies naturally between people - even with the same brand. Some absorb faster. Some slower. The 90% CI accounts for that biological noise without letting in unsafe differences.
And here’s another key point: both AUC and Cmax must pass. One can’t compensate for the other. If the generic delivers the same total exposure (AUC) but spikes too high (Cmax), it might cause side effects. If it’s slow to absorb (low Cmax), it might not work fast enough. Both matter.
Why This Rule Exists - And Why It’s Not Perfect
The 80-125% rule wasn’t born from a massive clinical trial. It came out of a 1986 FDA hearing, where experts looked at decades of data and said: differences under 20% in absorption are unlikely to cause real-world problems. It was a judgment call - not hard science. But over the last 40 years, it’s held up.
Post-market data backs it up. A 2020 FDA analysis of over 2,000 generic drugs approved between 2003 and 2016 found only 0.34% needed label changes due to bioequivalence issues after hitting the market. That’s less than 1 in 300.
Still, the rule has blind spots. For drugs with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - even a 10% difference can be dangerous. That’s why the FDA now recommends tighter limits: 90-111% for some of these drugs. The European Medicines Agency already uses this tighter range for certain products.
And then there are highly variable drugs. Some drugs, like certain antibiotics or epilepsy meds, show wildly different absorption from person to person. For these, the 80-125% range is too rigid. Regulators now use scaled average bioequivalence (SABE), which widens the acceptable range - up to 69.84-143.19% - based on how variable the brand drug is. This isn’t a loophole. It’s a smarter, data-driven adjustment.
Common Misconceptions - And Why They Matter
One of the biggest myths? That the 80-125% rule means generic drugs contain only 80% of the active ingredient. That’s wrong. The active ingredient in generics is usually within 95-105% of the brand - same as the brand itself. The rule is about absorption, not content.
A 2022 survey by the American Pharmacists Association found 63% of community pharmacists believed the rule meant generics had less drug in them. That’s dangerous. It fuels patient fear. On Reddit, Drugs.com, and Facebook groups, patients often refuse generics because they think they’re “only 80% strong.”
But here’s what those patients don’t see: the FDA’s Orange Book lists over 13,800 approved generic drugs. Nearly 90% of prescriptions in the U.S. are filled with generics. And if the 80-125% rule didn’t work, we’d see a flood of treatment failures. We don’t. We see savings - over $300 billion in the U.S. alone since 2009 - without a spike in hospitalizations or adverse events.
How the Rule Is Tested - And Who Gets It Right
Bioequivalence studies aren’t easy. They usually involve 24 to 36 healthy volunteers in a crossover design: each person takes the brand drug one time, the generic another, with a washout period in between. Blood is drawn over 24-72 hours. The data gets log-transformed. Confidence intervals are calculated. And both AUC and Cmax must pass.
It’s not just about crunching numbers. There are pitfalls. Some companies skip food-effect studies when they’re needed. Others don’t account for outliers properly. A study with more than 20% outlier data points needs a strong justification - or it gets rejected.
And then there’s the learning curve. A 2021 survey found only 38% of entry-level clinical researchers could correctly interpret a bioequivalence confidence interval without help. That’s why training programs like AAPS’s Bioequivalence Boot Camp exist - and why regulators keep updating guidance documents.

What’s Changing - And What’s Next
The 80-125% rule isn’t frozen in time. The FDA’s Complex Generics Initiative, launched in 2018, is tackling harder products: inhalers, topical creams, injectables, and extended-release pills. These don’t dissolve the same way as a tablet. Their absorption is trickier to measure. New methods - like in vitro-in vivo correlation (IVIVC) and model-informed bioequivalence - are being tested.
In 2021, the FDA expanded its bioequivalence waiver program. Some simple, immediate-release pills can now skip human studies if they meet strict dissolution criteria. That saves time and money - without compromising safety.
Looking ahead, pharmacogenomics could change the game. If your genes make you a slow metabolizer of a certain drug, should the same bioequivalence range apply? Maybe not. By 2030, we might see personalized bioequivalence limits based on genetic profiles - but that’s still years away.
For now, the 80-125% rule remains the backbone of generic drug approval worldwide. The FDA, EMA, WHO, and nearly every major regulator use it. It’s not perfect. But it’s proven. And for the millions who rely on affordable medicines, that’s what matters.
Does the 80-125% rule mean generic drugs have less active ingredient?
No. The 80-125% rule applies to how much of the drug enters your bloodstream - not how much is in the pill. Generic drugs must contain the same active ingredient as the brand, typically within 95-105% of the labeled amount. The rule measures absorption (AUC and Cmax), not content.
Why is a 90% confidence interval used instead of a 95%?
A 90% confidence interval allows for a 10% total error margin - 5% on each side. This balances the need for certainty with the reality that drug absorption naturally varies between people. A 95% CI would be too strict, blocking many safe and effective generics. The 90% CI has been shown to reliably predict clinical safety and effectiveness.
Are there drugs that need tighter bioequivalence limits?
Yes. Drugs with a narrow therapeutic index - like warfarin, levothyroxine, phenytoin, and digoxin - can have serious side effects from small changes in blood levels. For these, regulators often require a tighter range: 90-111%. The FDA and EMA apply these stricter limits case by case based on clinical risk.
What is scaled average bioequivalence (SABE)?
SABE is a method used for highly variable drugs - those where absorption varies a lot between people, even with the same brand. Instead of using a fixed 80-125% range, regulators adjust the acceptable limits based on how variable the reference drug is. For example, the range can expand to 69.84-143.19% for Cmax. This prevents safe drugs from being rejected just because they’re naturally unpredictable.
Do bioequivalence studies prove a generic works as well as the brand?
They don’t test clinical outcomes like seizure control or blood pressure reduction. Instead, they prove the generic delivers the same amount of drug to the bloodstream at the same rate as the brand. Over 40 years of post-market data show that generics approved under this standard perform just as well in real patients. Clinical trials aren’t needed because the absorption profile is the best predictor of effectiveness.
What This Means for You
If you’re taking a generic drug, you’re not getting a second-rate version. You’re getting a product that’s been held to the same rigorous standard as the brand - with one key difference: cost. The 80-125% rule is why generics are cheaper. It’s why you can fill a 30-day supply of metformin for $4 instead of $300.
It’s also why you should trust it. The rule isn’t magic. It’s math - refined by decades of data, real-world use, and expert review. When your pharmacist says, “This generic is bioequivalent,” they’re not guessing. They’re relying on a system that’s kept millions of people healthy for over 40 years.
And if you’re still unsure? Ask your doctor or pharmacist for the FDA’s Orange Book listing. Every approved generic is there. And every one passed the 80-125% test.