Bioequivalence: What It Means for Generic Drugs and Your Health
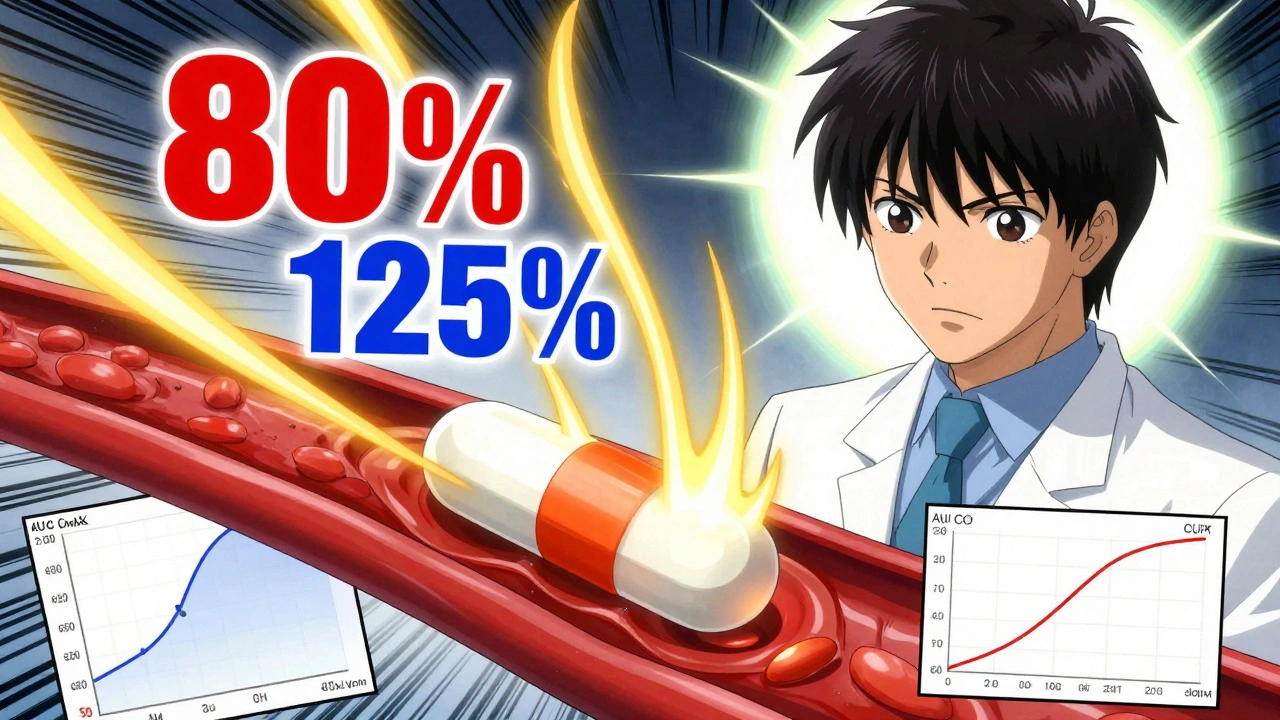
When you pick up a generic pill, you want to know it will do the same job as the brand-name version. That’s where bioequivalence, the scientific standard proving two drugs release the same amount of active ingredient at the same rate in the body. Also known as drug equivalence, it’s the reason your pharmacist can swap a brand-name drug for a cheaper generic without risking your health. The FDA doesn’t just accept claims—they test it. Bioequivalence studies measure how fast and how much of the drug enters your bloodstream. If the results fall within strict limits (usually 80% to 125% of the brand’s levels), the generic is approved. No guesswork. No shortcuts.
This isn’t just about cost—it’s about control. If you’re on a long-term medication like metformin or lisinopril, your body gets used to a steady dose. A drug that’s not bioequivalent could cause side effects, make your condition worse, or lead to dangerous spikes and drops in your system. That’s why recalls of generic drugs often trace back to manufacturing issues that break bioequivalence standards. It’s also why doctors and pharmacists rely on this data when switching patients to generics. The FDA approval, the official green light given after rigorous testing of a drug’s safety, strength, and bioequivalence isn’t a formality—it’s the final checkpoint before a generic hits the shelf.
Bioequivalence doesn’t mean identical packaging or taste. It means identical performance. A generic metformin extended-release tablet must release the drug slowly over hours, just like the brand. A generic tiotropium inhaler must deliver the same dose to your lungs. That’s why posts here cover everything from how generics are tested, to why some patients still hesitate to switch, to how patent laws and manufacturing locations can affect quality. You’ll find real stories about drug recalls, provider advice on switching meds, and how to spot when a generic isn’t working like it should. This isn’t theory—it’s daily reality for millions who depend on affordable meds.
Understanding bioequivalence gives you power. It lets you ask the right questions: Did this generic pass the same tests? Is there a recall? Why is this one cheaper than another? You don’t need to be a scientist to use this knowledge. You just need to know it matters. Below, you’ll find practical guides, real-world examples, and clear explanations that connect bioequivalence to your everyday health choices—whether you’re managing diabetes, high blood pressure, or just trying to save money without sacrificing safety.