Generic Drugs: What They Are, Why They Work, and How They Save You Money
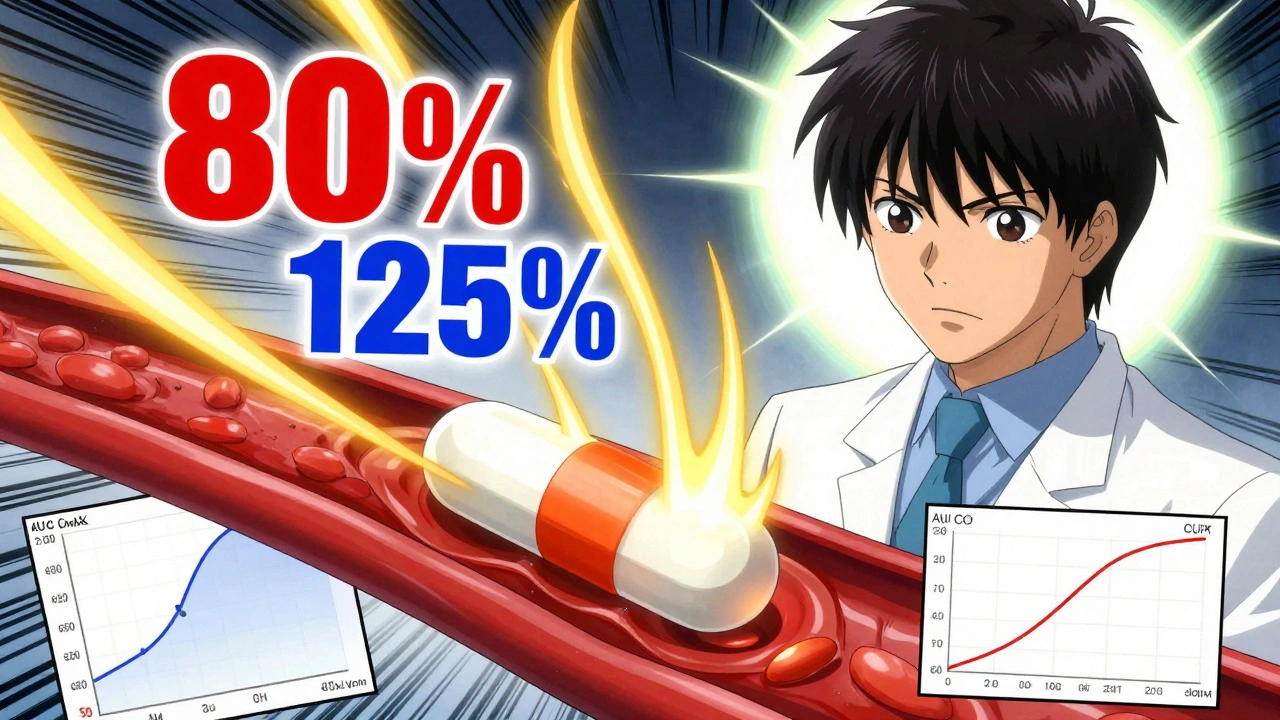
When you hear generic drugs, lower-cost versions of brand-name medications that contain the same active ingredients, meet the same safety standards, and work the same way in your body. Also known as generic medication, they’re not second-rate—they’re the backbone of affordable healthcare in Canada and around the world. The FDA and Health Canada require them to be bioequivalent to the original drug, meaning they deliver the same amount of medicine into your bloodstream at the same speed. That’s not marketing—it’s science. And it’s why millions of Canadians rely on generics for diabetes, high blood pressure, asthma, and more.
But not all generics are created equal in perception. Some people worry about quality because they’ve heard about generic drug recalls, occasional manufacturing issues, often linked to overseas production, that lead to contaminated or ineffective pills. Others wonder why their doctor switched them from a brand to a generic. The truth? Most doctors in Europe, Asia, and North America now recommend generics first—not because they’re cheap, but because they’re proven. Studies show no difference in outcomes for conditions like hypertension or depression when switching to generics. What changes is the price: a 30-day supply of a brand-name statin might cost $150, while the generic runs under $10. Over a lifetime, that adds up to tens of thousands saved.
Behind every generic drug is a complex system. The Hatch-Waxman Act, a U.S. law that balances patent protection with generic access by allowing the first generic maker to sell exclusively for 180 days after a patent expires triggered the biggest price drops in pharmaceutical history. That’s why the first generic version of a drug often slashes costs by 80% or more. But it’s not just about price. New rules now require bioequivalence studies to include older adults and women—not just young men—because age and sex can affect how a drug works. And while some people think generics are less effective, the real issue is often side effects: a different filler or coating might cause stomach upset where the brand didn’t. That’s why knowing your options matters.
You’ll find posts here that break down how generics are tested, why recalls happen, and how to spot a bad batch. You’ll see how providers talk to patients about switching, what happens when a first generic hits the market, and how international doctors view these drugs. You’ll also learn how to save even more by syncing refills, avoiding dangerous interactions with supplements, and knowing when a combination pill might do more harm than good. This isn’t just about saving money—it’s about taking control. If you’re on long-term meds for cholesterol, thyroid, or diabetes, you’re already using generics. The question isn’t whether they work. It’s whether you’re getting the best value—and the safest version—for your health.